Why aprevo® Personalized Spine Devices?
Your spine is unique—and your treatment should be too. aprevo® combines personalized design, AI-enabled planning, and breakthrough technology to create spinal implants built specifically for you.


Why aprevo®?
We are all different and unique. Your spine implant should be, too.
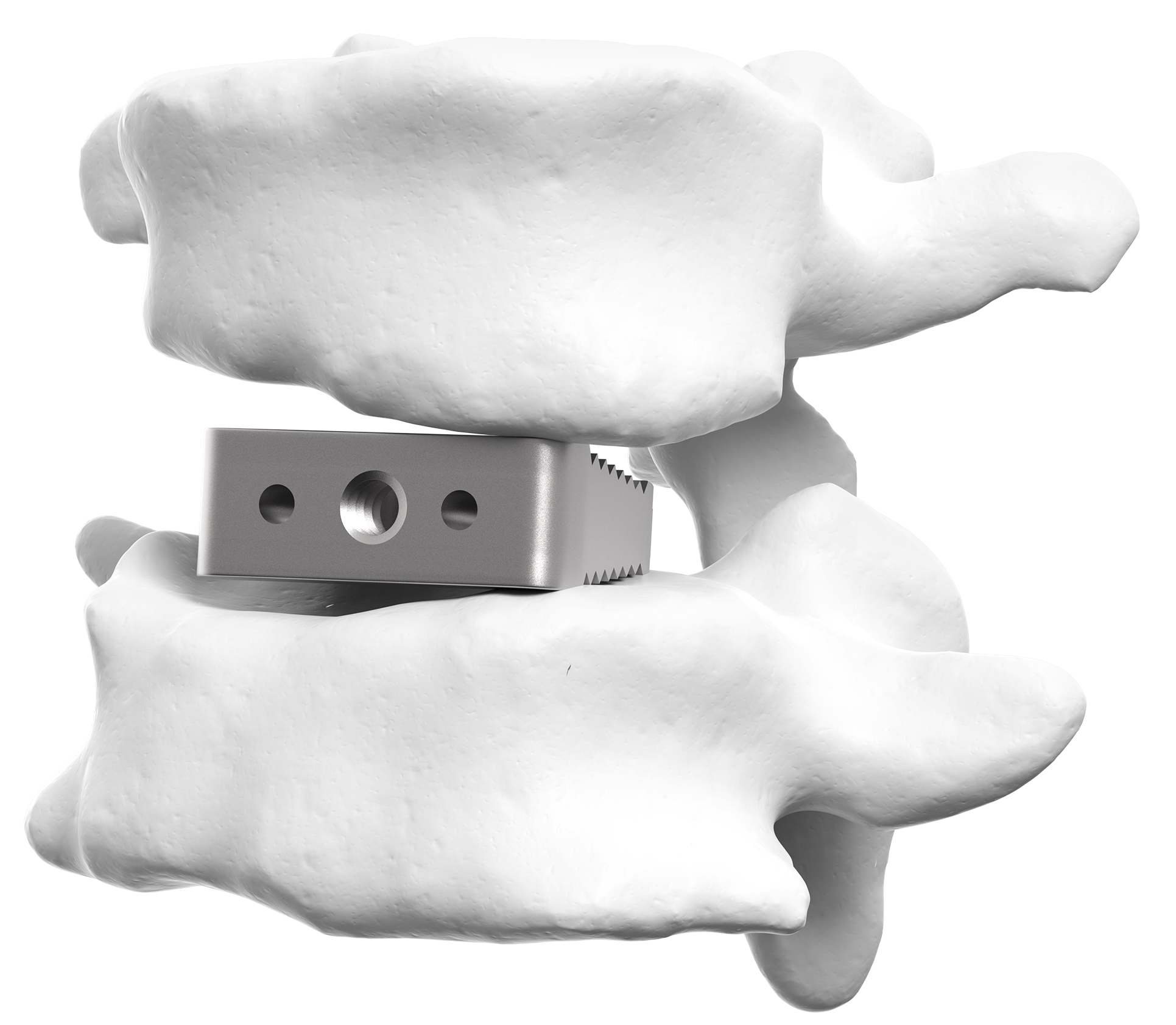
The premier personalized solution for spinal fusion

The only FDA designated Breakthrough Technology for intervertebral spine fusion

AI-enabled surgical planning

aprevo® personalized implants are designed to provide for a more effective treatment vs. standard implants.

aprevo® Personalized Implant
Standard Implant
Fewer
Revisions
Improved Spinal
Alignment
82% of personalized aprevo® interbody levels achieved targeted alignment within 5°.2
Improved Bone
Graft Contact
At one year, CT scans showed aprevo® devices with an average bone contact surface area of 94%.3
We are all different and unique.
Your spine implant should be, too.
The aprevo® Personalized Spine Procedure is the only intervertebral spine implant that is personalized for you.
Unique. Innovative. Breakthrough.
Spine surgery is very personal. aprevo® AI-enabled surgical planning and patient-specific implants are a breakthrough approach to creating a personalized solution specifically for you.
References:
- 1. Kent RS, Ames CP, Asghar J, et al. Radiographic alignment in deformity patients treated with personalized interbody devices: early experience from the COMPASS registry. Int J Spine Surg. 2024;18(S1):S6-S15. doi:10.14444/8636
- 2. Sadrameli SS, Blaskiewicz DJ, Asghar J, et al. Predictability in achieving target intervertebral lordosis using personalized interbody implants. Int J Spine Surg. 2024;18(S1):S16-S23. doi: 10.14444/8637
- 3. Ames C, Smith J, Nicolau R. Tomographic assessment of fusion rate, implant-endplate contact area, subsidence, and alignment with lumbar personalized interbody implants at one-year follow-up. Intl J of Spine Surg. 2024; 18(S1):S41-S49. doi.org/10.14444/8640

