The aprevo® personalized spine procedure is the first available solution to provide personalized digital surgical plans and the accompanying aprevo® interbody implants that are tailored to each patient.
The FDA Breakthrough Device Designation
Unique. Innovative. Breakthrough.
aprevo® is the first intervertebral spine implant to receive FDA Breakthrough Device Designation as well as the Transformative New Technology designation from the Centers for Medicare & Medicaid Services (CMS).
Furthermore, we received FDA 510(k) clearance for our aprevo® Technology Platform for cervical spine fusion surgery.
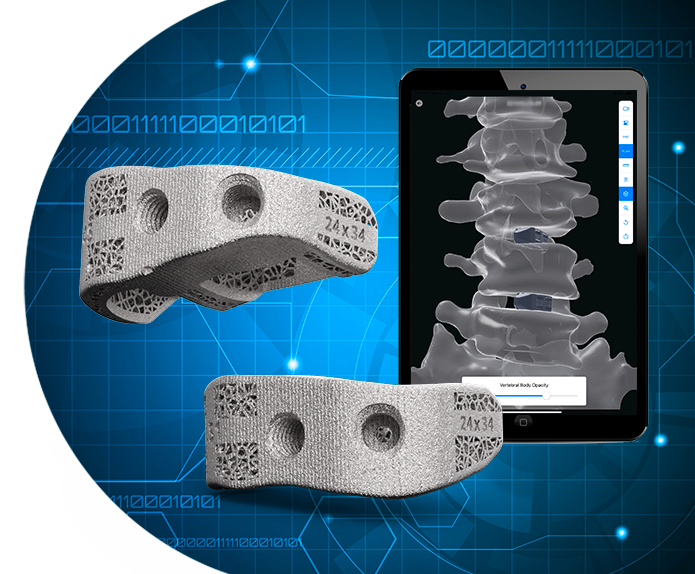
We are all different and unique. Your spine implant should be, too.
It all begins with YOU.
Talk to your doctor about the aprevo® patient-specific spinal implants, or find a doctor with our surgeon locator.






CONNECT WITH US